Thermodynamics and statistical mechanics
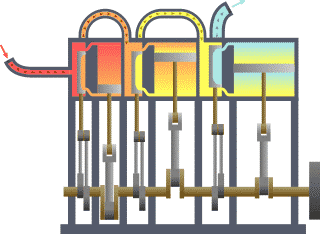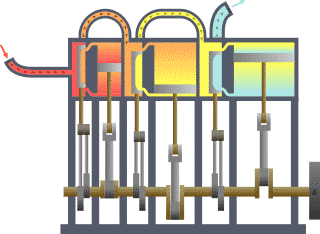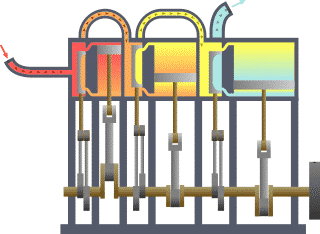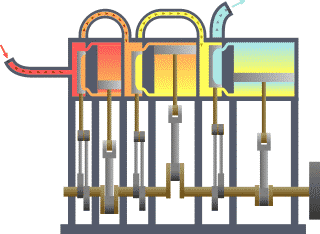
Thermodynamics studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic scale, and the transfer of energy as heat.[13][14] Historically, thermodynamics developed out of need to increase the efficiency of early steam engines.[15]
The starting point for most thermodynamic considerations are the laws of thermodynamics, which postulate that energy can be exchanged between physical systems as heat or work.[16] They also postulate the existence of a quantity named entropy, which can be defined for any system.[17] In thermodynamics, interactions between large ensembles of objects are studied and categorized. Central to this are the concepts of system and surroundings. A system is composed of particles, whose average motions define its properties, which in turn are related to one another through equations of state. Properties can be combined to express internal energy and thermodynamic potentials, which are useful for determining conditions for equilibrium and spontaneous processes.
Statistical mechanics analyzes macroscopic systems by applying statistical principles to their microscopic constituents. It provides a framework for relating the microscopic properties of individual atoms and molecules to the macroscopic or bulk properties of materials that can be observed in everyday life. Thermodynamics can be explained as a natural result of statistics and mechanics (classical and quantum) at the microscopic level. In this way, the gas laws can be derived, from the assumption that a gas is a collection of individual particles, as hard spheres with mass. Conversely, if the individual particles are also considered to have charge, then the individual accelerations of those particles will cause the emission of light. It was these considerations which caused Max Planck to formulate his law of blackbody radiation,[18] but only with the assumption that the spectrum of radiation emitted from these particles is not continuous in frequency, but rather quantized.[19]
No comments:
Post a Comment